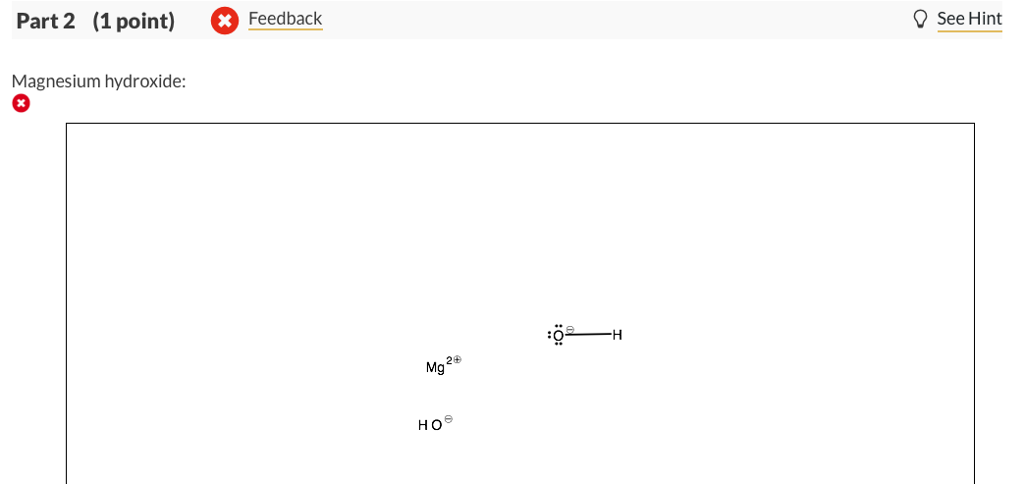
For calcium carbonate draw both the cation and the anions as standalone ions. Draw the most common Lewis structure, and do not draw alternative resonance forms.
By A Mystery Man Writer
Last updated 14 May 2024


a) The cyanate ion, OCN-, forms a number of stable salts, but many fulminates, CNO-, are explosive. Draw the possible Lewis structures for both anions and explain this reactivity difference. b) Nitro

chem exam 2 Flashcards
Solved Part1 (1point) x Feedbaclk ad See Periodic Table See

OneClass: For each part draw both the the cation and the anions as standalone ions. For each organic
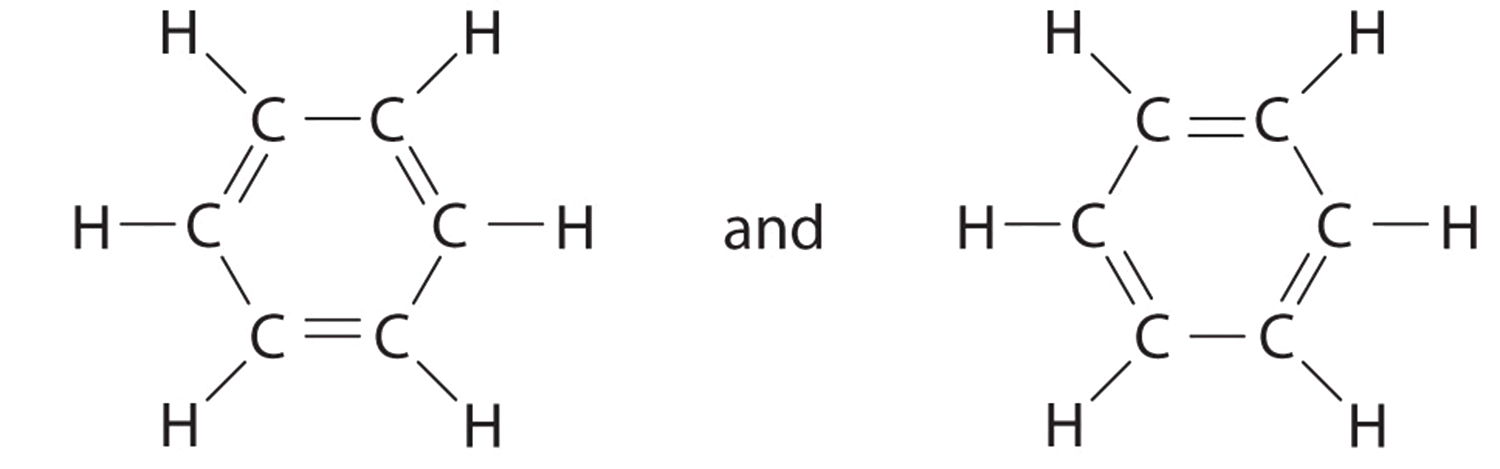
8.6: Resonance Structures - Chemistry LibreTexts

Antacid tablets commonly contain calcium carbonate and/or magnesium hydroxide. Draw the Lewis structures for calcium carbonate and magnesium hydroxide.

How to Draw the Lewis Dot Structure for CaI2: Calcium iodide

Soil Organic Matter Characterization by Fourier Transform Ion Cyclotron Resonance Mass Spectrometry (FTICR MS): A Critical Review of Sample Preparation, Analysis, and Data Interpretation
8.24 For the carbonate ion, CO3 2−, draw all of the resonance structures. Identify which orbitals
Recommended for you
-
 NOW Supplements, Calcium Carbonate Powder, High Percentage of Calcium, Supports Bone Health* 12-Ounce14 May 2024
NOW Supplements, Calcium Carbonate Powder, High Percentage of Calcium, Supports Bone Health* 12-Ounce14 May 2024 -
 Calcium Carbonate, Fine ground limestone, FCC14 May 2024
Calcium Carbonate, Fine ground limestone, FCC14 May 2024 -
 What is Calcium Carbonate?14 May 2024
What is Calcium Carbonate?14 May 2024 -
 Why Should Calcium Carbonate be Surface Coated?14 May 2024
Why Should Calcium Carbonate be Surface Coated?14 May 2024 -
 CAS Number 471-34-114 May 2024
CAS Number 471-34-114 May 2024 -
 Buy Calcium Carbonate Online Calcium Carbonate Latest Price14 May 2024
Buy Calcium Carbonate Online Calcium Carbonate Latest Price14 May 2024 -
 a Unit cell of calcium carbonate (calcite, CaCO3) showing partial14 May 2024
a Unit cell of calcium carbonate (calcite, CaCO3) showing partial14 May 2024 -
 30+ Thousand Calcium Carbonate Royalty-Free Images, Stock Photos & Pictures14 May 2024
30+ Thousand Calcium Carbonate Royalty-Free Images, Stock Photos & Pictures14 May 2024 -
 Calcium Carbonate (Calcite)14 May 2024
Calcium Carbonate (Calcite)14 May 2024 -
 Magnesium Impurities Decide the Structure of Calcium Carbonate Hemihydrate14 May 2024
Magnesium Impurities Decide the Structure of Calcium Carbonate Hemihydrate14 May 2024
You may also like
-
 12 Count Pokemon Pencil Favors, Multicolor : : Toys14 May 2024
12 Count Pokemon Pencil Favors, Multicolor : : Toys14 May 2024 -
 Why does Miracle Whip exist? Leave it to us Americans to fuck up mayonnaise. : r/StupidFood14 May 2024
Why does Miracle Whip exist? Leave it to us Americans to fuck up mayonnaise. : r/StupidFood14 May 2024 -
 Reversible 5mm Sequin Fabric by the Yard - OneYard14 May 2024
Reversible 5mm Sequin Fabric by the Yard - OneYard14 May 2024 -
 Custom Embroidered Patches, Winston-Salem, NC14 May 2024
Custom Embroidered Patches, Winston-Salem, NC14 May 2024 -
Lilo & Stitch : Toys for Ages 0-24 Months : Target14 May 2024
-
 120 Pcs Sew on Rhinestones for Clothes Glass Rhinestones 10-18 mm Big Rhinestones Claw Flatback Prong Setting Sew on Crystal Sewing Gems for DIY14 May 2024
120 Pcs Sew on Rhinestones for Clothes Glass Rhinestones 10-18 mm Big Rhinestones Claw Flatback Prong Setting Sew on Crystal Sewing Gems for DIY14 May 2024 -
 Floss Drops 'Farm Fresh Eggs' by Lori Holt. Store Cross Stitch Thread while stitching. Its Sew Emma product. USA Import. Fat Quarter Shop – The Stitchery Dorset14 May 2024
Floss Drops 'Farm Fresh Eggs' by Lori Holt. Store Cross Stitch Thread while stitching. Its Sew Emma product. USA Import. Fat Quarter Shop – The Stitchery Dorset14 May 2024 -
 Custom Game Pieces Manufacturer, Design & Create Board Game Pieces14 May 2024
Custom Game Pieces Manufacturer, Design & Create Board Game Pieces14 May 2024 -
:quality(70):extract_cover():upscale():fill(ffffff)/2020/04/29/740/n/1922153/60d44da3321a456b_netimgoz2cSj.jpg) Tula The Cult Classic Purifying Face Cleanser Review14 May 2024
Tula The Cult Classic Purifying Face Cleanser Review14 May 2024 -
![AntiqueGoldPatina [Stained glass]](https://i.ytimg.com/vi/ofN-jtEkC_4/maxresdefault.jpg) AntiqueGoldPatina [Stained glass]14 May 2024
AntiqueGoldPatina [Stained glass]14 May 2024
