Characterization and Stability Study of Polysorbate 20 in Therapeutic Monoclonal Antibody Formulation by Multidimensional Ultrahigh-Performance Liquid Chromatography–Charged Aerosol Detection–Mass Spectrometry
By A Mystery Man Writer
Last updated 18 May 2024


Characterization and Stability Study of Polysorbate 20 in Therapeutic Monoclonal Antibody Formulation by Multidimensional Ultrahigh-Performance Liquid Chromatography–Charged Aerosol Detection–Mass Spectrometry

Effect of Polysorbate 20 and Polysorbate 80 on the Higher-Order Structure of a Monoclonal Antibody and Its Fab and Fc Fragments Probed Using 2D Nuclear Magnetic Resonance Spectroscopy. - Abstract - Europe PMC

Characterization and Stability Study of Polysorbate 20 in Therapeutic Monoclonal Antibody Formulation by Multidimensional Ultrahigh-Performance Liquid Chromatography–Charged Aerosol Detection–Mass Spectrometry

Understanding Particle Formation: Solubility of Free Fatty Acids as Polysorbate 20 Degradation Byproducts in Therapeutic Monoclonal Antibody Formulations

Simultaneous quantification of polysorbate 20 and poloxamer 188 in biopharmaceutical formulations using evaporative light scattering detection - ScienceDirect
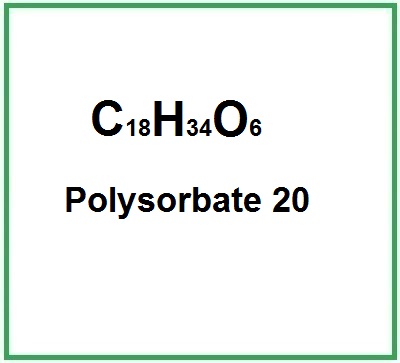
Polysorbate 20

Evaluation of the In-Use Stability of Monoclonal Antibody IV Admixtures Prepared from Drug Products Containing Polysorbate 20 Degraded by Host-Cell Lipases - Journal of Pharmaceutical Sciences

Understanding Mixed-Mode Retention Mechanisms in Liquid Chromatography with Hydrophobic Stationary Phases
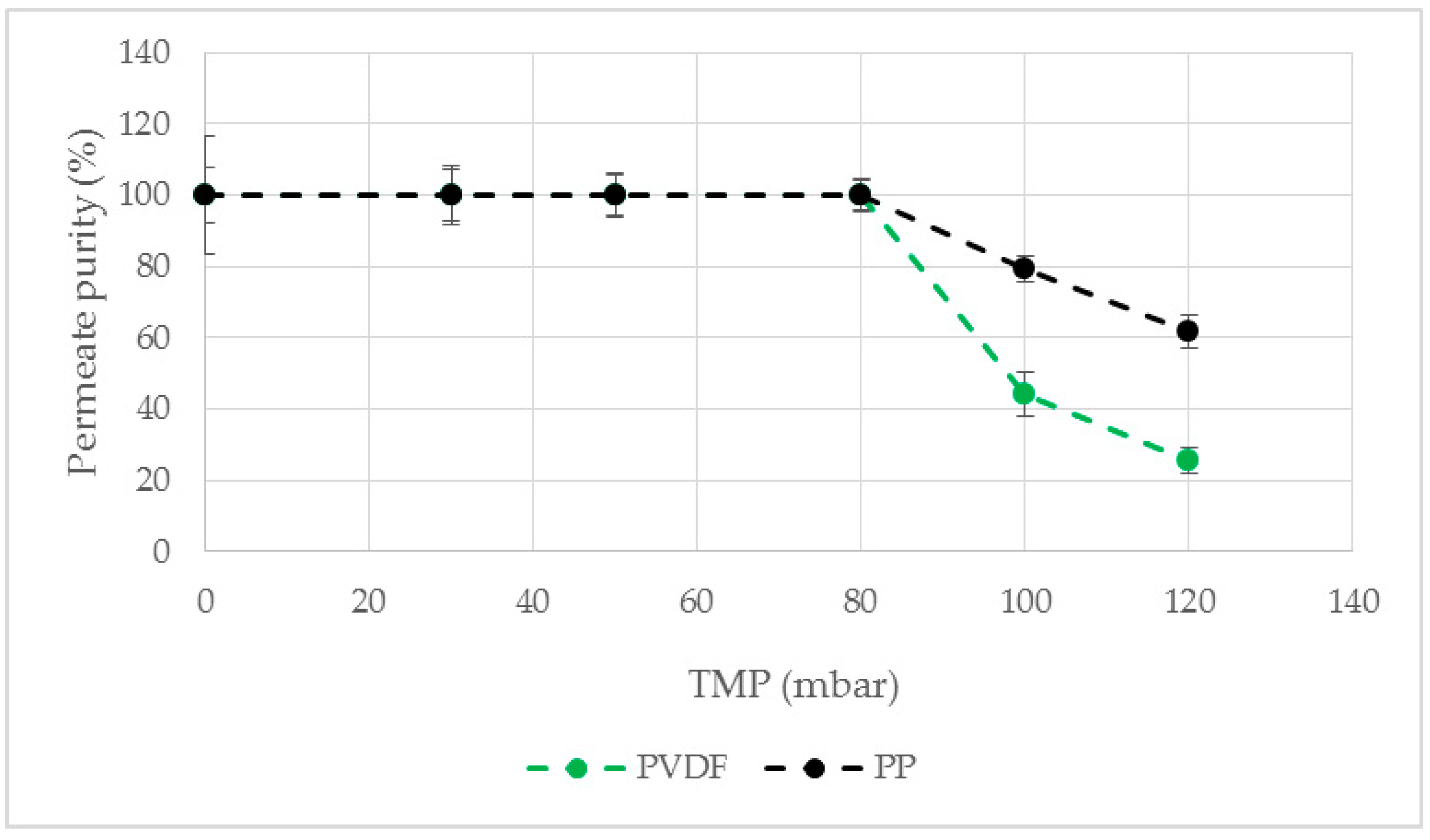
Antibodies, Free Full-Text
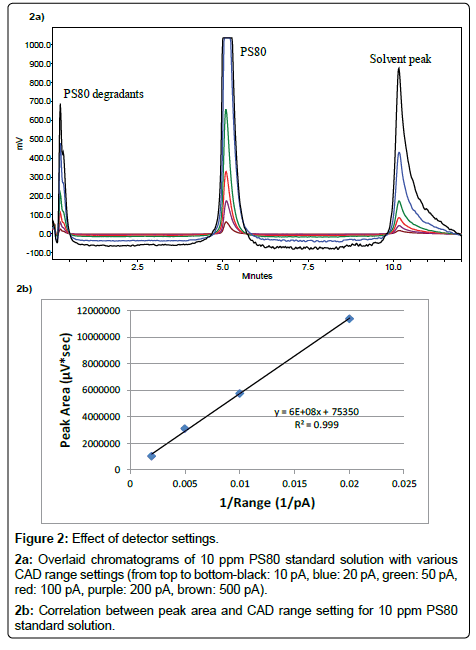
A Highly Sensitive Method for the Quantitation of Polysorbate 20 and 80 to Study the Compatibility between Polysorbates and m-Cresol in the Peptide Formulation

Characterization and Stability Study of Polysorbate 20 in Therapeutic Monoclonal Antibody Formulation by Multidimensional Ultrahigh-Performance Liquid Chromatography–Charged Aerosol Detection–Mass Spectrometry

Characterization of polydisperse macrogols and macrogol‐based excipients via HPLC and charged aerosol detection

Characterization of polydisperse macrogols and macrogol‐based excipients via HPLC and charged aerosol detection
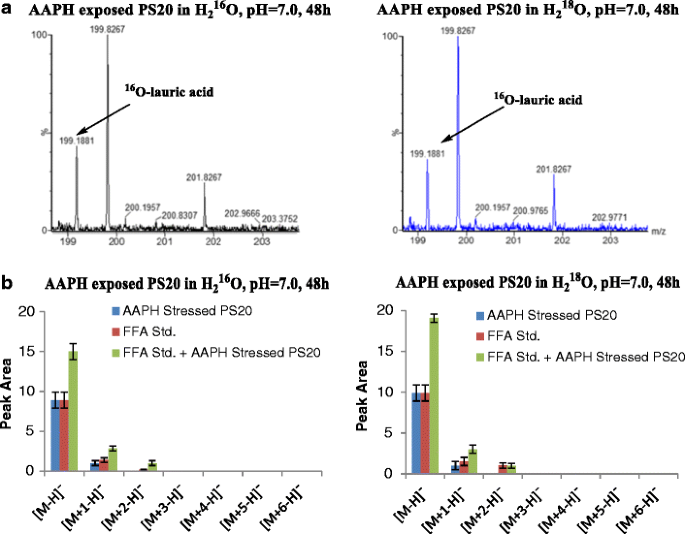
Degradation Mechanisms of Polysorbate 20 Differentiated by 18O-labeling and Mass Spectrometry
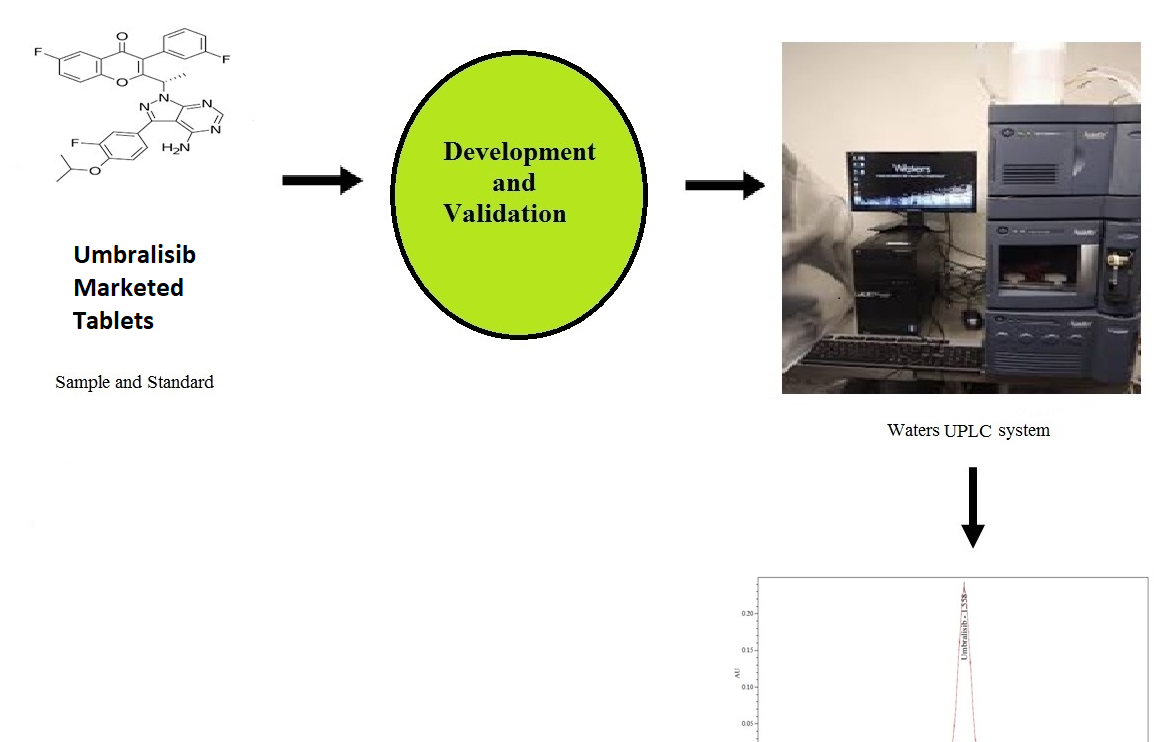
Determination of Umbralisib using reverse phase ultra performance liquid chromatography in bulk and pharmaceutical dosage form
Recommended for you
-
 Polysorbate 20, liquid18 May 2024
Polysorbate 20, liquid18 May 2024 -
 Tween® 2018 May 2024
Tween® 2018 May 2024 -
 Polysorbate 2018 May 2024
Polysorbate 2018 May 2024 -
 Polysorbate 20 - an overview18 May 2024
Polysorbate 20 - an overview18 May 2024 -
 Polysorbate-20-NF, CAS 9005-64-5, PO13218 May 2024
Polysorbate-20-NF, CAS 9005-64-5, PO13218 May 2024 -
-500x500.jpg) Tween 20 (Non-Ionic Detergent), CAS 9005-64-5, Polysorbate 2018 May 2024
Tween 20 (Non-Ionic Detergent), CAS 9005-64-5, Polysorbate 2018 May 2024 -
 Hydrolysis of Polysorbate 20 and 80 by a Range of Carboxylester Hydrolases18 May 2024
Hydrolysis of Polysorbate 20 and 80 by a Range of Carboxylester Hydrolases18 May 2024 -
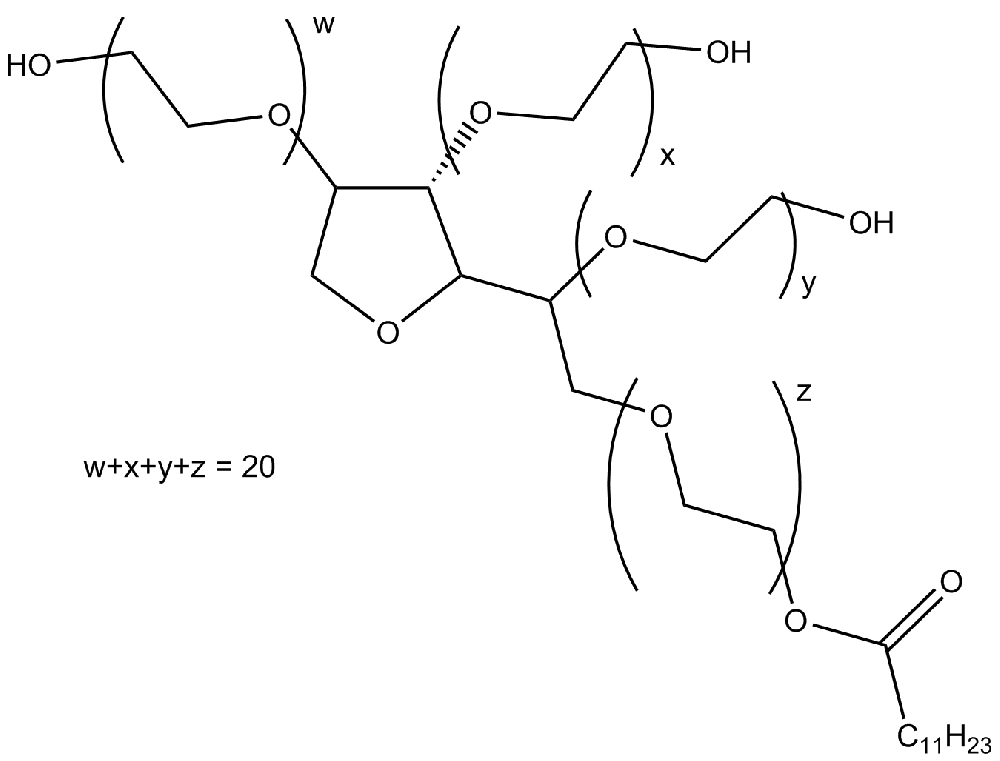 Polyoxyethylenesorbitan (Tween® 20)18 May 2024
Polyoxyethylenesorbitan (Tween® 20)18 May 2024 -
 Myoc Polysorbate 20 - 100ml18 May 2024
Myoc Polysorbate 20 - 100ml18 May 2024 -
 Polysorbate 20 in Skin Care: Why Is It Bad for Your Skin18 May 2024
Polysorbate 20 in Skin Care: Why Is It Bad for Your Skin18 May 2024
You may also like
-
 Wrapping Paper Thread Card Scissors Ribbon High-Res Stock Photo - Getty Images18 May 2024
Wrapping Paper Thread Card Scissors Ribbon High-Res Stock Photo - Getty Images18 May 2024 -
 11/0 Clear/Seafoam Green Line Seed Bead-0629-9018 May 2024
11/0 Clear/Seafoam Green Line Seed Bead-0629-9018 May 2024 -
 No Nonsense Agile Podcast a podcast by Murray Robinson & Shane Gibson18 May 2024
No Nonsense Agile Podcast a podcast by Murray Robinson & Shane Gibson18 May 2024 -
 How to Draw Cute Things for Kids: A Fun and Easy Step-By-Step18 May 2024
How to Draw Cute Things for Kids: A Fun and Easy Step-By-Step18 May 2024 -
 Daruma 29-color sashiko gift set18 May 2024
Daruma 29-color sashiko gift set18 May 2024 -
 It's Just - Citric Acid, Food Grade, Non-GMO, Bath Bombs (2.5 Pounds) : Beauty & Personal Care18 May 2024
It's Just - Citric Acid, Food Grade, Non-GMO, Bath Bombs (2.5 Pounds) : Beauty & Personal Care18 May 2024 -
:upscale()/2019/11/27/788/n/1922441/d735b35e5ddeb8b0e753d5.08363279_.jpg) Pandora Harry Potter Charm Collection, 202018 May 2024
Pandora Harry Potter Charm Collection, 202018 May 2024 -
 New Dabo & Shobo Markers for Sale in Arrowhed Farm, CA - OfferUp18 May 2024
New Dabo & Shobo Markers for Sale in Arrowhed Farm, CA - OfferUp18 May 2024 -
 Wavy Arch Clay Earring Cutter, Scalloped Arch Cutter, Clay Cutters18 May 2024
Wavy Arch Clay Earring Cutter, Scalloped Arch Cutter, Clay Cutters18 May 2024 -
Febreze Free Fabric Refresher Spray 27 fl oz 0.8 quart 4 Carton18 May 2024
