EAS Consulting Group - Specializing in FDA Regulatory Matters
By A Mystery Man Writer
Last updated 26 May 2024


EAS Consulting Group, LLC

Pharma's Problems with Data Integrity - EAS Consulting Group

Sanitary Design, Certification And FSMA Compliance, 60% OFF

How to Successfully Respond to FDA 483s and Warning Letters

EAS Consulting Group

EAS Consulting Group (@EAS_Consulting) / X

21 CFR 111 GMP Laboratory Overview (1 of 5 GMP Compliance in DS Laboratories Series)

1 Emerging Issues in Food Safety Dr Kalpagam Polasa*, Ph. D. Scientist 'F' & HoD FDTRC and Dr. B. Sesikeran*, MD, FAMS Director *National Institute of. - ppt download

EAS Consulting Group - Specializing in FDA Regulatory Matters

EAS Consulting Group - Specializing in FDA Regulatory Matters
Recommended for you
-
 UPDATED 11/28): Sage Users Receive FCC Deadline Extension – Colorado Broadcasters Association26 May 2024
UPDATED 11/28): Sage Users Receive FCC Deadline Extension – Colorado Broadcasters Association26 May 2024 -
 EAS (Emergency Alert System), Object Shows Community26 May 2024
EAS (Emergency Alert System), Object Shows Community26 May 2024 -
 EAS Simulator by Lexian-droid - Game Jolt26 May 2024
EAS Simulator by Lexian-droid - Game Jolt26 May 2024 -
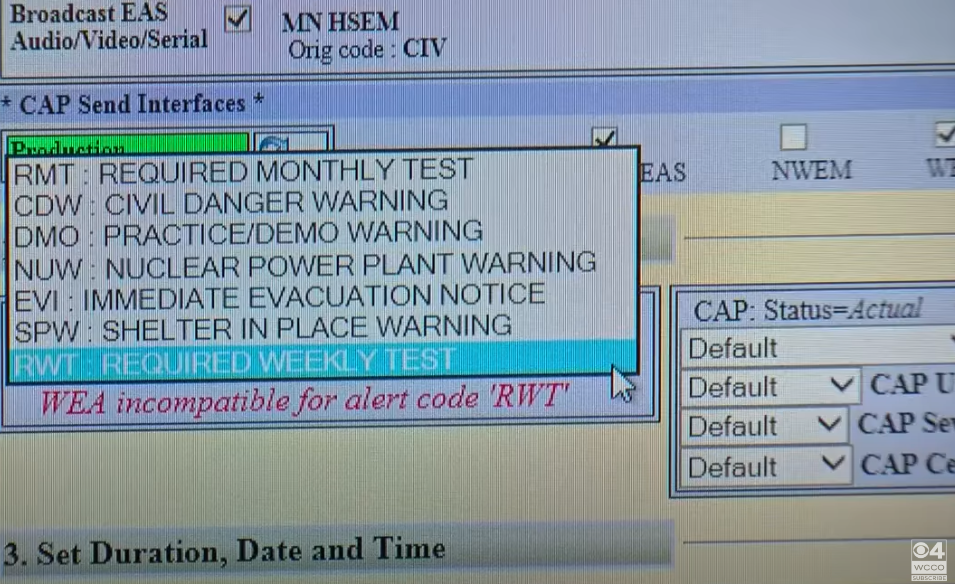 Sounding the Alarm on Emergency Alert System Flaws – Krebs on Security26 May 2024
Sounding the Alarm on Emergency Alert System Flaws – Krebs on Security26 May 2024 -
 EAS Systems: Advantages for Retailers26 May 2024
EAS Systems: Advantages for Retailers26 May 2024 -
 Emergency Alert System/EAS font : r/identifythisfont26 May 2024
Emergency Alert System/EAS font : r/identifythisfont26 May 2024 -
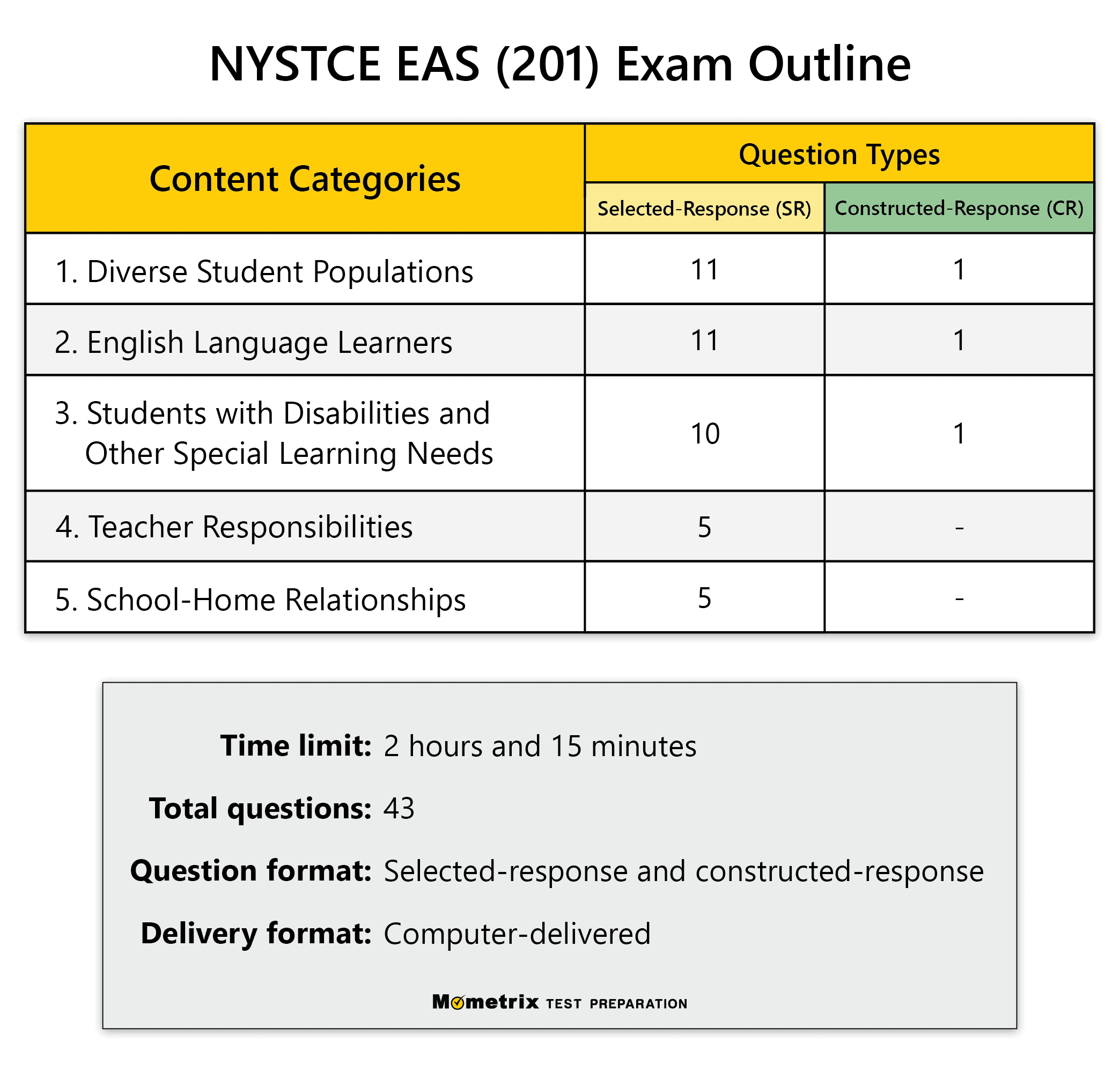 Free NYSTCE EAS Educating All Students Test Practice Test (updated 2024)26 May 2024
Free NYSTCE EAS Educating All Students Test Practice Test (updated 2024)26 May 2024 -
 EAS, Products26 May 2024
EAS, Products26 May 2024 -
Screens of the EAS, Emergency Alert System Wiki26 May 2024
-
 EAS 100% Whey Protein Powder, Chocolate, 30g Protein, 2 lb26 May 2024
EAS 100% Whey Protein Powder, Chocolate, 30g Protein, 2 lb26 May 2024
You may also like
-
 Copper Powder Atomized Metal - Weight: 100g - by Inoxia INX23326 May 2024
Copper Powder Atomized Metal - Weight: 100g - by Inoxia INX23326 May 2024 -
 Lucalab Composition Pencil Pen Tray26 May 2024
Lucalab Composition Pencil Pen Tray26 May 2024 -
 12 Grids 3D Nail Charms Rhinestones Bow Heart Nail Art Gems Decoration Wedding26 May 2024
12 Grids 3D Nail Charms Rhinestones Bow Heart Nail Art Gems Decoration Wedding26 May 2024 -
 How to Make a 12x12 Scrapbooking Album ~ Mintay Precious Moments26 May 2024
How to Make a 12x12 Scrapbooking Album ~ Mintay Precious Moments26 May 2024 -
 Bare Cadena26 May 2024
Bare Cadena26 May 2024 -
 5 Creative Scrapbook for Couple Ideas - JK Crafts For the creative in you26 May 2024
5 Creative Scrapbook for Couple Ideas - JK Crafts For the creative in you26 May 2024 -
 Smile Face Beads, 10mm Happy Face, Acrylic, Cute26 May 2024
Smile Face Beads, 10mm Happy Face, Acrylic, Cute26 May 2024 -
 Disney Stitch Edible Icing Cupcake or Cookie Decor Toppers - STH126 May 2024
Disney Stitch Edible Icing Cupcake or Cookie Decor Toppers - STH126 May 2024 -
 30+ easy DIY sea glass crafts · VickyMyersCreations26 May 2024
30+ easy DIY sea glass crafts · VickyMyersCreations26 May 2024 -
 3 Pcs Set 2 Ways Silicone Nail Art Acrylic Pen Brushes Set Blue Rhinestones Design Sculpture Carving Brush For 3d Effect Shaping Drawing Dotting Tools26 May 2024
3 Pcs Set 2 Ways Silicone Nail Art Acrylic Pen Brushes Set Blue Rhinestones Design Sculpture Carving Brush For 3d Effect Shaping Drawing Dotting Tools26 May 2024
