Development and validation of a selective marker-based quantification of polysorbate 20 in biopharmaceutical formulations using UPLC QDa detection - Pharma Excipients
By A Mystery Man Writer
Last updated 18 Jun 2024
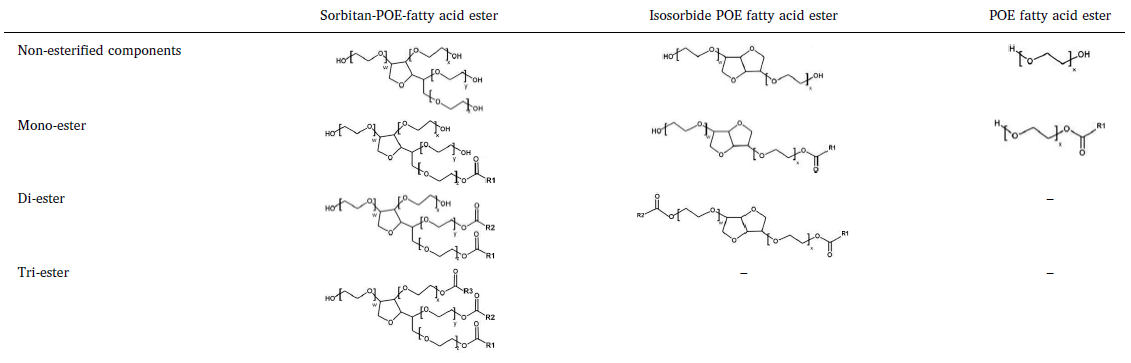
Polysorbates are widely used as non-ionic surfactant in biopharmaceutical formulations. Recently, the degradation of polysorbate moved into the focus of
Polysorbates are widely used as non-ionic surfactant in biopharmaceutical formulations. Recently, the degradation of polysorbate moved into the focus of attention, because in several published studies it was described, that stability issues in polyso
Polysorbates are widely used as non-ionic surfactant in biopharmaceutical formulations. Recently, the degradation of polysorbate moved into the focus of attention, because in several published studies it was described, that stability issues in polyso
RaDes EN Archive - Page 2 of 2 - RaDes GmbH

Polysorbate 20 Degradation in Biopharmaceutical Formulations: Quantification of Free Fatty Acids, Characterization of Particulates, and Insights into the Degradation Mechanism
RaDes EN Archive - Page 2 of 2 - RaDes GmbH

Quantitative Analysis of Polysorbate 20/80 in Protein-Based Biopharmaceuticals Using A One-Pot RPLC-MS Based Platform Method

Characterization and Stability Study of Polysorbate 20 in Therapeutic Monoclonal Antibody Formulation by Multidimensional Ultrahigh-Performance Liquid Chromatography–Charged Aerosol Detection–Mass Spectrometry
promotercatalystimage-1700816724160.jpg
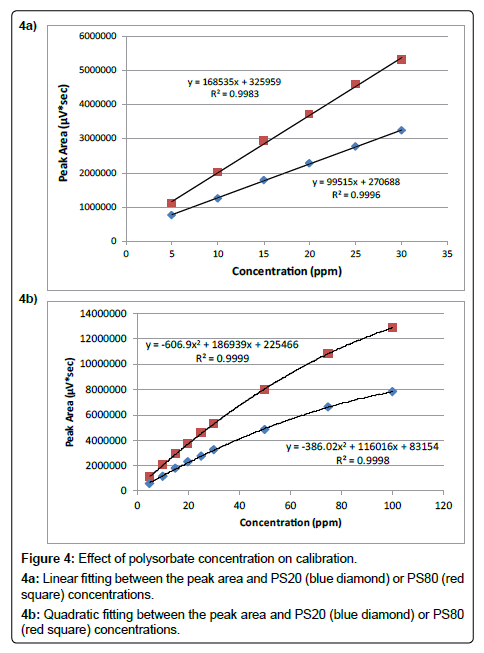
A Highly Sensitive Method for the Quantitation of Polysorbate 20 and 80 to Study the Compatibility between Polysorbates and m-Cresol in the Peptide Formulation
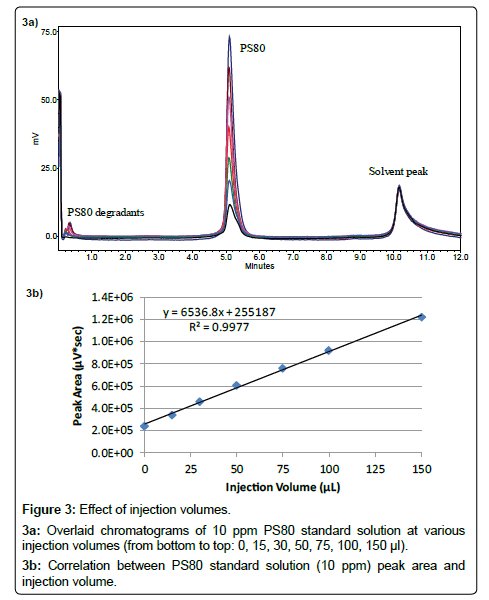
A Highly Sensitive Method for the Quantitation of Polysorbate 20 and 80 to Study the Compatibility between Polysorbates and m-Cresol in the Peptide Formulation
RaDes EN Archive - RaDes GmbH

Polysorbate 20 Degradation in Biopharmaceutical Formulations: Quantification of Free Fatty Acids, Characterization of Particulates, and Insights into the Degradation Mechanism

Polysorbate 20 Degradation in Biopharmaceutical Formulations: Quantification of Free Fatty Acids, Characterization of Particulates, and Insights into the Degradation Mechanism

Development of a High-Throughput Formulation Screening Platform for Monoclonal AntibodiesBioProcess International

Hydrolytic polysorbate 20 degradation – Sensitive detection of free fatty acids in biopharmaceuticals via UPLC-QDa analytics with isolator column - ScienceDirect
Recommended for you
-
 Polysorbate 20 T-MAZ 20 Tween 20 Surfactant Emulsifier18 Jun 2024
Polysorbate 20 T-MAZ 20 Tween 20 Surfactant Emulsifier18 Jun 2024 -
 Tween 20, CAS 9005-64-5, Viscous Liquid18 Jun 2024
Tween 20, CAS 9005-64-5, Viscous Liquid18 Jun 2024 -
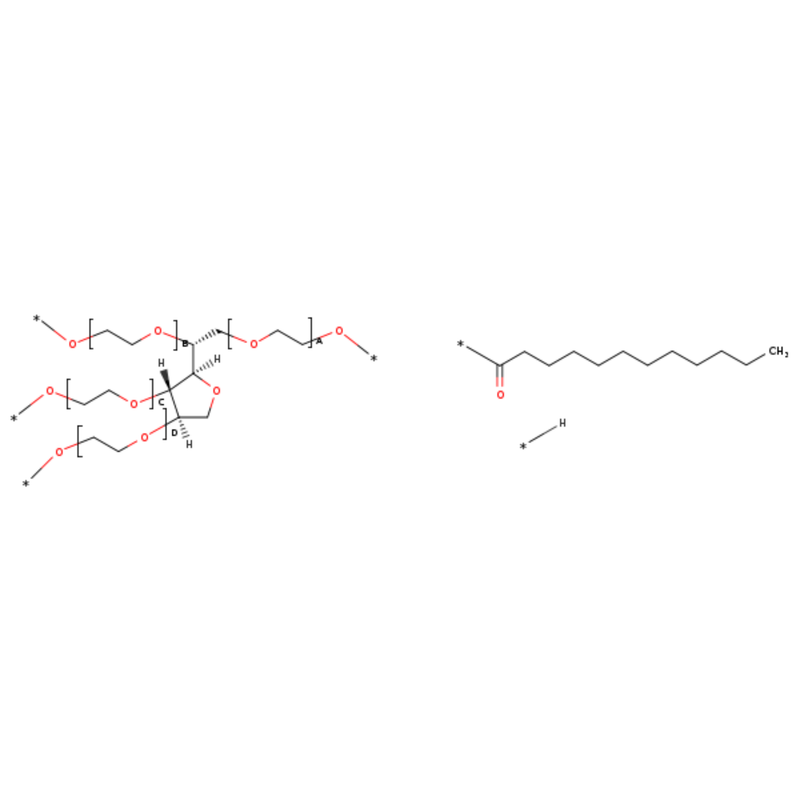 Polysorbate 20 - ALODERMA18 Jun 2024
Polysorbate 20 - ALODERMA18 Jun 2024 -
 Differences between polysorbate 20 and polysorbate 80 - Guangdong Huana Chemistry Co., Ltd.18 Jun 2024
Differences between polysorbate 20 and polysorbate 80 - Guangdong Huana Chemistry Co., Ltd.18 Jun 2024 -
 Polysorbate 20 by Velona - 2 oz, Solubilizer, Food & Cosmetic Grade, All Natural for Cooking, Skin Care and Bath Bombs18 Jun 2024
Polysorbate 20 by Velona - 2 oz, Solubilizer, Food & Cosmetic Grade, All Natural for Cooking, Skin Care and Bath Bombs18 Jun 2024 -
 Polysorbate-20 (Tween 20)18 Jun 2024
Polysorbate-20 (Tween 20)18 Jun 2024 -
 Polysorbate 20 - 2 fl oz18 Jun 2024
Polysorbate 20 - 2 fl oz18 Jun 2024 -
 Perfume Studio Polysorbate 20 Solubilizer Cosmetic Grade18 Jun 2024
Perfume Studio Polysorbate 20 Solubilizer Cosmetic Grade18 Jun 2024 -
 Differences between polysorbate 20 and polysorbate 80 - Guangdong18 Jun 2024
Differences between polysorbate 20 and polysorbate 80 - Guangdong18 Jun 2024 -
 Velona Polysorbate 20 Solubilizer, Food, Cosmetic, Grade, Cooking18 Jun 2024
Velona Polysorbate 20 Solubilizer, Food, Cosmetic, Grade, Cooking18 Jun 2024
You may also like
-
 KUCHT KRH3001U Under Cabinet Range Hood 30 inch18 Jun 2024
KUCHT KRH3001U Under Cabinet Range Hood 30 inch18 Jun 2024 -
 Fall Floral & Vines Square Punch Needle Kit - Gather Goods Co.18 Jun 2024
Fall Floral & Vines Square Punch Needle Kit - Gather Goods Co.18 Jun 2024 -
 Large Dish Drying Rack, 2-Tier Dish Racks for Kitchen Counter18 Jun 2024
Large Dish Drying Rack, 2-Tier Dish Racks for Kitchen Counter18 Jun 2024 -
 INTBUYING Vinyl 24 Cutting Plotter Heat Press Transfer Machine Vinyl Cutter With Vinyls KIT18 Jun 2024
INTBUYING Vinyl 24 Cutting Plotter Heat Press Transfer Machine Vinyl Cutter With Vinyls KIT18 Jun 2024 -
 Artificial Topiary Ball Lifelike Plants Rose - Temu18 Jun 2024
Artificial Topiary Ball Lifelike Plants Rose - Temu18 Jun 2024 -
 XILYZMO Cork Board Sheet Paper Roll, Curved Fold in Cork Boards, with Adhesive-Backed Wall Decoration Cork Roll, Easy to Cut Penetrati Onsmoothly Noticeboard (Color : 0.11in, Size : 48x 118in)18 Jun 2024
XILYZMO Cork Board Sheet Paper Roll, Curved Fold in Cork Boards, with Adhesive-Backed Wall Decoration Cork Roll, Easy to Cut Penetrati Onsmoothly Noticeboard (Color : 0.11in, Size : 48x 118in)18 Jun 2024 -
 How to Make a Pleated Seat Cover for a Motorcycle18 Jun 2024
How to Make a Pleated Seat Cover for a Motorcycle18 Jun 2024 -
 I was just looking around my room when i found that fake action figure of nightmare foxy, what should i do with it? : r/fivenightsatfreddys18 Jun 2024
I was just looking around my room when i found that fake action figure of nightmare foxy, what should i do with it? : r/fivenightsatfreddys18 Jun 2024 -
 Бумага упаковочная крафт Мокрый тропический лес, 0,7 х 10 м, 70 г (3562875) - Купить по цене от 299.00 руб.18 Jun 2024
Бумага упаковочная крафт Мокрый тропический лес, 0,7 х 10 м, 70 г (3562875) - Купить по цене от 299.00 руб.18 Jun 2024 -
 Groz Beckert Industrial Sewing Machine Needles DBx1 1738 16x257 R DB1 All Sizes18 Jun 2024
Groz Beckert Industrial Sewing Machine Needles DBx1 1738 16x257 R DB1 All Sizes18 Jun 2024